Download this note (PDF)
What is the difference between cell culture media and spent media?
Cell culture involves growth of cells in an artificial environment. Cell culture medium is a liquid designed to support the growth of cells in this environment. Usually, it contains a mixture of
- an energy source like carbohydrates in the form of sugars;
- amino acids since they are the building blocks of proteins;
- fatty acids and lipids are particularly important in serum-free media;
- inorganic salts to maintain osmotic balance;
- vitamins and trace elements essential for growth and proliferation of cells;
- buffering system to maintain the pH in the physiological range.
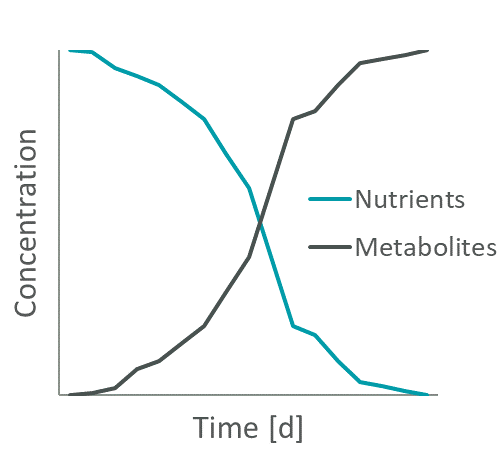
Figure 1: Exemplary change in nutrient and metabolite levels during cell cultivation.
Spent media is the cell culture media that is formed by cell growth and remains after culturing of cells. During cell growth both nutrient depletion and metabolite accumulation occur in the medium (e.g., Figure 1). And last but not least, there is also the target product that the cells have produced, which can, in certain circumstances, also become toxic if concentrations become too high.
Spent media analysis is the analysis of the used media over the course of the production process and facilitates the selection of the right cell culture medium and feed combination, as well as the development of suitable feeding strategies. It is a useful tool that enables us to gain insight into the metabolic processes as well as to track and understand changes in medium composition. At Xell we do spent media analysis in four stages, ranging from basic insight into full process understanding (c.f. Table 1):
- Analysis of only primary parameters like pH value, osmolality, glucose, lactate, glutamine and ammonium.
- Analysis of essential parameters like amino acids, dipeptides, and vitamins.
- For a comprehensive understanding of the bioprocess, we measure and analyze trace elements and ions.
- Analysis for in-depth understanding of the bioprocess involves in addition to the above, measurement of e.g., organic acids, polyamines, and nucleotides.
Why should we do spent media analysis?
Since different mammalian cells have different features the media used to culture them should be designed for optimal growth of each cell type or clone. Limitations occur when the composition of the used cell culture medium does not match the cells’ requirements. An ill-suited medium could underfeed or overfeed the cells. The depletion of nutrients and the accumulation of growth inhibitory metabolites can cause cessation of cell growth. Oversupply of substrates can lead to unwanted overflow metabolism and inefficient use of provided nutrients. In addition, accumulating waste metabolites negatively impact process parameters and performance of cultures. Therefore, regular monitoring of changes in sugars, metabolites, vitamins and amino acids is done to optimize feed strategies as well as harvest times and select appropriate cell culture medium, all to promote high cell viability, maximize production of titer, and ensure high product quality.
Case study: Selecting optimal feed strategy
See box for description of most common feeding strategies in bioprocessing. Typically, mammalian cells are glycolytic and will produce lactate from glucose in the medium. In Figure 2 we see that glucose levels go down and are depleted in the batch samples. By detecting this limitation of glucose by spent media analysis it is possible to design an optimized feeding strategy for a fed-batch process where feed supplements are added periodically.
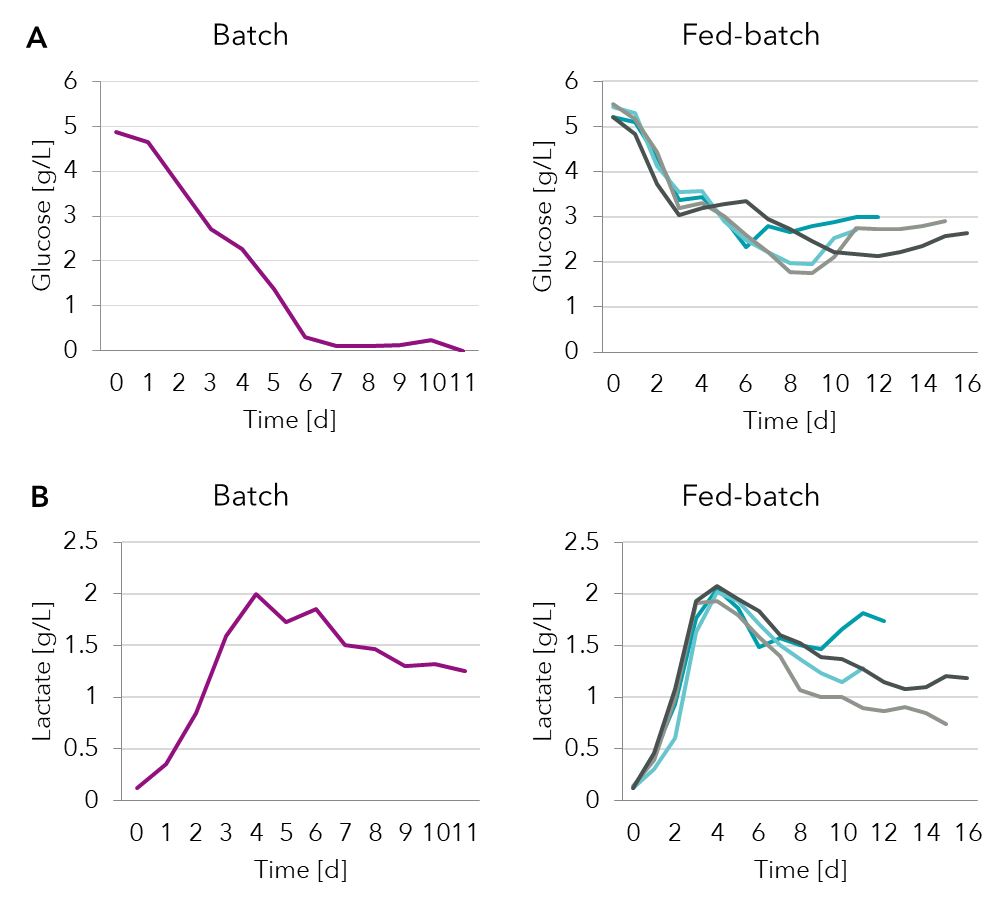
Figure 2: Spent media analysis of (A) glucose and (B) lactate in a batch and several fed-batch processes.
As glycolysis proceeds lactate levels increase in both batch and fed-batch processes. Eventually after about 4 days the lactate levels stabilize. This is due to a metabolic switch when the cells transition from a lactate producing to a lactate consuming culture. This metabolic switch called the lactate shift is a desirable characteristic. Accumulation of lactate would limit cell growth but switching to lactate consumption would prevent acidification of the medium and not slow cell growth.
Analysis of select amino acids from the spent media showed (Figure 3) that both asparagine and serine were depleted by day 6/8 in batch process. Fed-batch supplementation strategies were able to recover serine levels better than asparagine. The fed-batch strategy (dark grey) that slowed down depletion of both asparagine and serine achieved better production (Figure 4B).
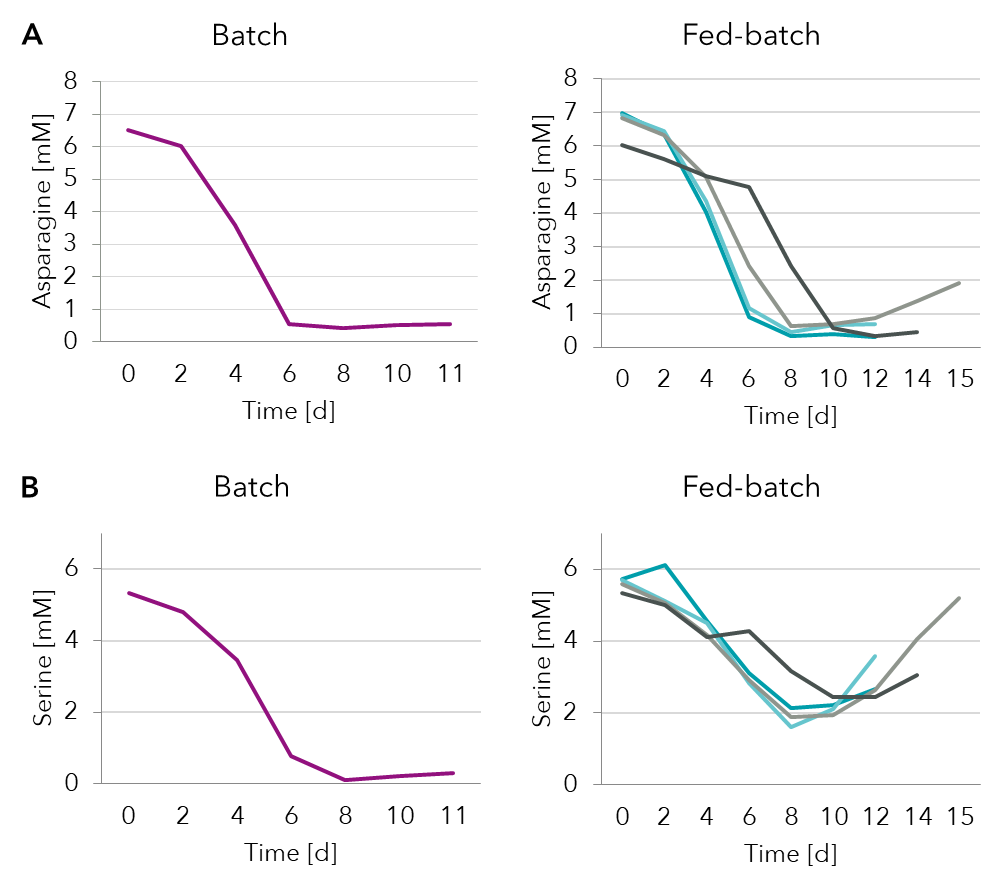
Figure 3: Spent media analysis of amino acids asparagine and serine in a batch and several fed-batch processes.
Analysis of the spent media for antibody titer (Figure 4C) and productivity (Figure 4B) show that cell specific productivity was best for fed-batch process 1 (dark blue) but the maximal antibody titer was achieved with fed-batch process 4 (dark grey). Further modification of feeding strategy by monitoring glucose, lactate, asparagine and serine levels lead to an increase of overall process yield through higher cell density and prolonged process while cell specific productivity was maintained at +20% compared to batch process. This shows how spent media analysis can aid in significant titer increase.
The examples shown here looked only at glucose, lactate, asparagine and serine levels in the spent media. A full panel of spent media analysis is required for samples taken all throughout the production run in order to optimize the feed for optimal bioprocess yield.
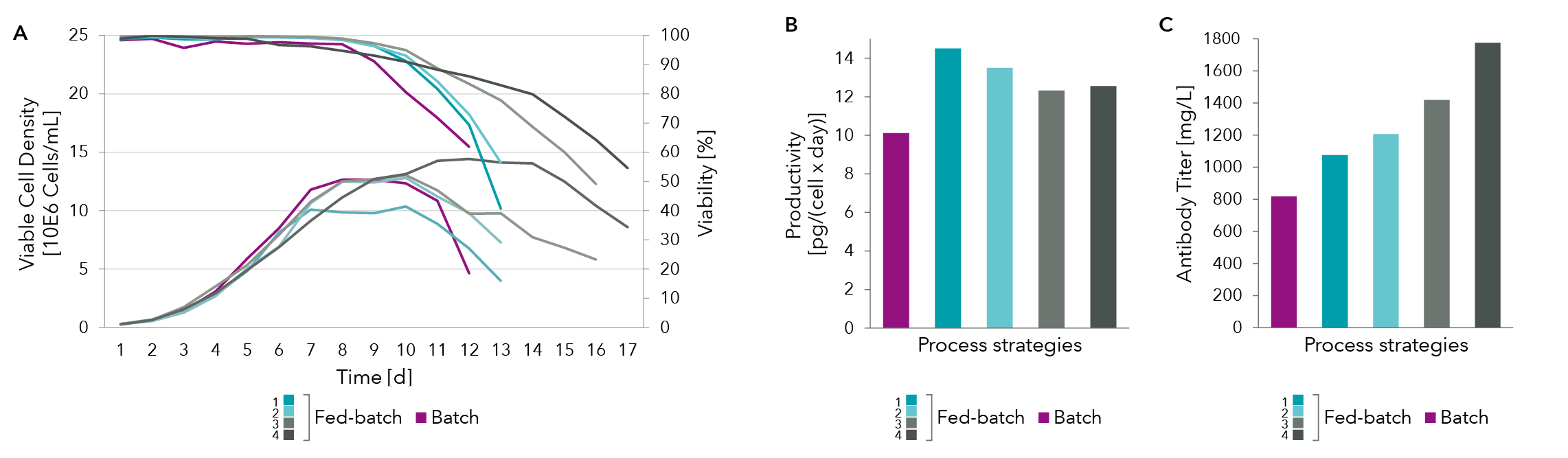
Figure 4: (A) Cell density as well as (B) titer and productivity measures for various feed strategies.
Cell culture process formats
A batch process is inoculated with the medium of choice and no further nutrients are added during the cultivation.
In a fed-batch process, additional substrates or supplements are added to increase process duration, cell density and productivity. The feed supplement can be added in bolus or continuous mode. The feeding strategy should be adapted to the cell’s requirements which can be initially determined using spent media analysis.
Continuous processes can be performed with (perfusion mode) and without cell retention (chemostat mode). Fresh nutrients are continuously added to the culture while spent medium (and cells) are harvested at the same time. With this process strategy, toxic metabolites can be removed and the process is especially suited for unstable products.
Spent media analysis available at Xell
Using our long-standing experience in cell culture media development we have established fast and robust methods for measuring the concentrations of a variety of components in cell culture media with our state-of-the-art equipment (c.f. Table 1).
Table 1: Spent media analysis for various components.
| Proteins, peptides, amino acids | |
| Dipeptides | UHPLC-MS/MS |
| Free amino acids | UHPLC-DAD |
| Total amino acids | UHPLC-DAD |
| Vitamins | |
| B Vitamins | UHPLC-MS/MS |
| Ions and Elements | |
| Ammonium | Multimodereader |
| Ions | ICP-MS |
| Phosphate | Multimodereader |
| Trace elements | ICP-MS |
| Sugars | |
| Glucose | Chip-Sensor |
| Lactate | Chip-Sensor |
| Others | |
| Organic acids | UHPLC-MS |
| Polyamines | UHPLC-MS/MS |
| And many more | … |
If you have questions regarding spent media analysis or if you want to gain insight into your processes by having your spent media analyzed, feel free to contact us directly and discuss your individual analytical needs with us or take a first look at our culture media analysis bundle, a pre-composed set of parameters specifically selected for spent media.
You do not want to miss any of these interesting insights and news? Subscribe to our newsletter here.






