Download this note (PDF)
Shaken cultures of suspension-adapted animal cell lines are still the method of choice for screening and small-scale culture experiments due to their easy handling, the simple and fast implementation in almost any laboratory environment and the possibility of cost-effective parallelization. However, on closer inspection, there are a few points that need to be considered and which play a major role in successful cultivation. These include:
- the supply of oxygen to the cells
- the impact of shear stress
- a physiological pH value
- a defined and steady temperature
While modern cell culture media and incubators take some of these points away from the experimenter, the oxygen supply and shear forces are directly influenced by the mode of shaking, specifically shaking speed (rpm) and deflection. The latter is often overlooked when implementing specifications for new cell lines, new media or testing setups provided by colleagues, clients, contractors, or even the literature. In everyday laboratory life, there is often no time or resources available for large-scale empirical tests or the planned screening does not justify the additional effort. So what can be done if the test or given specifications require a completely different shaking platform (in terms of deflection) than is currently available? As trivial as this question may seem, it often determines the success of a test and many media products cannot develop their full potential under suboptimal conditions.
Impact of deflection on cell cultivation
As discussed, cell growth and other cellular processes such as transfection efficiency depend not only on a sufficient supply of nutrients but also on temperature, shaker type, shaker size, filling volume, shaking speed and deflection of the shaking platform. If these parameters are not optimally adjusted to the respective cell system and the media used, reduced cell growth, aggregation or even death of the cell culture is often the result. Even if cell growth seems to be consistent, processes such as transfection can be impacted as well (see Figure 1). If excessive shear forces act on cells due to an excessively high shaking speed, which may also not be balanced by the optimal concentration of surface-active agents (surfactants, e.g. Pluronic® F-68 / Kolliphor® P 188) in the medium, the growth rate, viability and/or e.g. transfection efficiency decreases rapidly. If, on the other hand, the shaking speed is too low, the cells may sediment and also die due to e.g. the reduced oxygen input. This factor depends not only on the available surface (keyword: filling volume!) but also on the mixing of the culture.
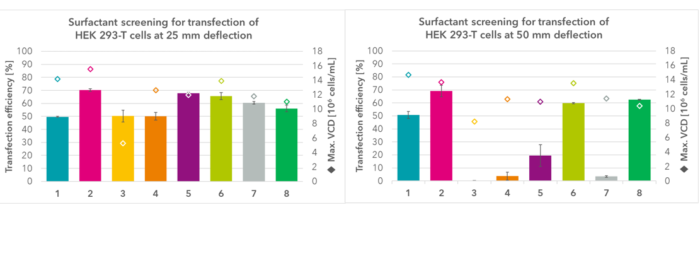
Figure 1: Exemplary data showing the impact of different shaker deflection (25 mm and 50 mm, both set to 185 rpm) for transient gene expression in a HEK cell line using eight media with different surfactants and concentrations thereof. While maximum cell densities seem to be relatively stable, transfection efficiencies are gravely impacted by increased centrifugal forces at 50 mm deflection (depending on the medium).
While the optimal shaking speed itself depends mainly on the shaker size and filling volume as well as the medium used and should be determined (empirically) for each cell, the transfer between shaking platforms with different deflections can be achieved relatively easily.
Successful cultivations, (almost) independent of deflection
The most common deflections of orbital shaking platforms for shaking flasks in cell culture are 25 mm and 50 mm. Figure 2 below illustrates the relationship between shaking speed and relative centrifugal force at different deflections. This makes it easy to estimate the appropriate shaking speed directly in the laboratory without taking the filling volume and the type of shaker into account. It is important here that the relative centrifugal force remains constant at different deflections. The cells should then be observed over a few passages and the speed should be corrected slightly upwards or downwards depending on growth and viability.
Alternatively, the new shaking speed can also be determined mathematically. For this purpose, the equations below are required. For special shapes of shaking flasks differing from the well-known Erlenmeyer bottles, large filling volumes or large-volume shakers, other factors may have to be taken into account.
Equation 1 describes the mathematical relationship between the shaking speed [rpm], the relative centrifugal force RCF [×g] and the rotor radius R [mm] at room temperature.
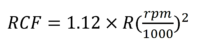
Equation 2 is used to calculate the new shaking speed, where r1 [rpm] describes the current shaking speed at a given deflection d1 [mm] and r2 [rpm] the shaking speed to be calculated at the target deflection d2 [mm].
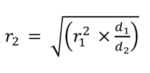
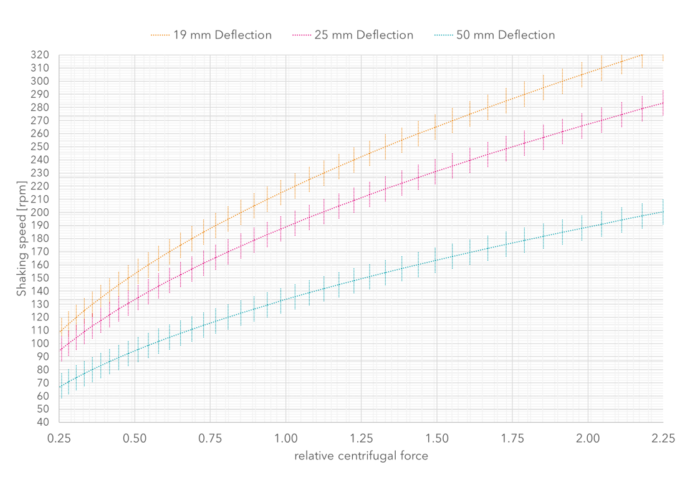
Figure 2: Diagram for determining the shaking speeds at different deflections for plain Erlenmeyer shake flasks. For determination, starting from the existing deflection and shaking frequency, go to the desired deflection vertically on the line of equal relative centrifugal force and then read off the corresponding shaking speed on the y-axis. For example, cultivation is currently taking place at 185 rpm and 50 mm deflection, which corresponds to a relative centrifugal force of 1.9×g, and in the future, it is planned to switch to a 25 mm deflection. At constant relative centrifugal force this corresponds to a shaking speed of 262 rpm.
Still in trouble with your culture? We are happy to help, just visit us!
Find out about Xell’s Cell Culture Services






