Download this note (PDF)
Fruit are full of vitamins and very healthy, so their juices are healthy too. That is something most people were told since they were little. We do not want to doubt that, but not all vitamins are stable, therefore the question is: Does juice age?
We went to a local grocery store to find out.
Multi juices and their vitamins
For a first experiment we bought two different multi vitamin juices and one multi juice. They all had different fruit or juices as ingredients. Amongst them: Apples, oranges, bananas, mangos and others. The multi vitamin juices also list different vitamins as ingredients, whereas the multi juice only states vitamin C as ingredient. This indicates that the multi vitamin juices are artificially vitamin enriched, while the multi juice is not. Additionally, we tested the vitamin content over time. Samples taken at three different time points were measured directly after sampling at 0 h, 48 h and 120 h, respectively.

After we opened the juices for the 0 h measurement, one aliquot was stored at 4°C in the dark and another aliquot was stored at room temperature next to a window. All samples were stored in 50 ml reaction tubes. Therefore, it was possible to not only compare the vitamin contents over time, but also the storage conditions.
Results
We measured different vitamins and vitaminoids using Xell’s proprietary method for water soluble vitamins (details can be found here).
The results displayed in Figure 1) focus on vitamins that showed remarkable concentration changes over time. Other vitamins were either not present or were not affected during our 120 h period. For instance, panthotenate shows to be rather unaffected and is displayed as example.
Overall, juice B showed a higher vitamin content, as compared to juice A and juice C. While juices A and B are called multi vitamin juices, juice C is called multi juice. This difference in the naming was also clearly visible in the measurements, since juice C did not show a multi vitamin content. Juices A and B were quite alike in their vitamin composition, only riboflavin (vitamin B2) is exclusively present in juice A. This is in accordance with the ingredient list.
Biotin was detected in both multi vitamin juices, however a difference in storage conditions was clearly visible. While biotin showed to be stable at 4°C in the dark, its concentration in both, juice A and B, decreased during the 120 h time period when stored at room temperature next to a window. Similar results were obtained for thiamine (vitamin B1). As mentioned before, riboflavin was only detected in juice A. At 4°C in the dark it was stable for 48 h and showed a decreased concentration at 120 h. The sample that was stored next to a window at room temperature barely contained riboflavin after 48 h and even less after 120 h. This clearly indicates the instability of vitamin B2 at unfavorable storage conditions. Rather different results were obtained when nicotinamide was analyzed. Regardless of the storage conditions, nicotinamide seemed stable for 48 h and then showed a severe decrease.
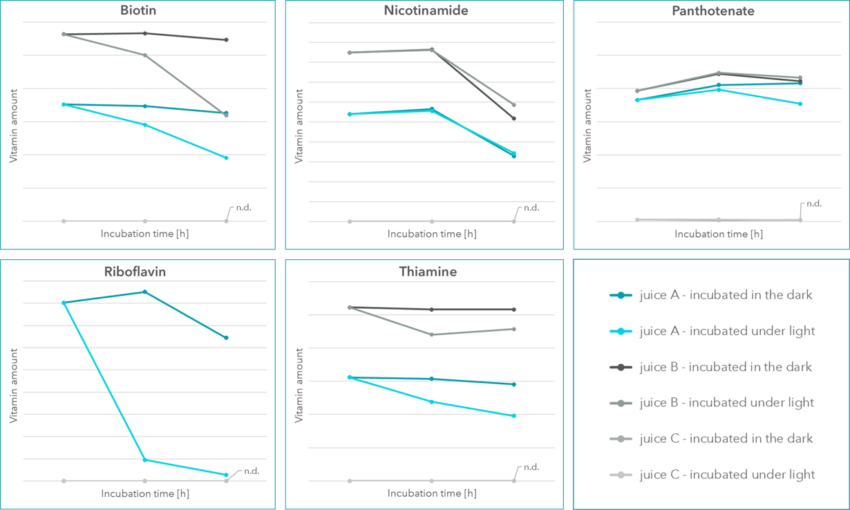
Figure 1: Values of different vitamins originating from multi juices, measured over a 120 h period. Each sample was measured as duplicate.
Conclusion
Do not expect too many vitamins, if your juice is called multi and not multi vitamin juice. Furthermore, keep in mind that not all vitamins are stable. Their content can be maintained better, if the juices are correctly stored. Overall, if you want the most out of the juices vitamins, drink up right after opening it.
An orange juice a day… For one week?
While we bought multi juices like described above, an orange juice explicitly advertising a vitamin C content caught our attention. Truly, oranges are often associated with vitamin C and that needed to be tested as well. We bought that orange juice and took a sample. Additionally, we squeezed an orange to get fresh juice and measured the vitamin C contents in parallel. Furthermore, we left both juices in the fridge (4°C, dark) for a week and an aliquot of the commercial juice next to a window, too. The stability of ascorbate and the ability to reconstitute it was tested.
Results
We measured vitamin C (ascorbate) in its reduced state with Xell’s proprietary method. After one week of storage an aliquot was measured “as is” and another aliquot was treated with a reducing agent.
Interestingly, the vitamin C contents of the freshly opened commercial juice and the squeezed orange, were quite alike (Figure 2). After one week the vitamin C content in all samples was noticeably reduced. The sample that was stored close to a window at room temperature barley contained vitamin C after a week of storage. The samples from the dark fridge still contained some vitamin C, with the sample from the squeezed orange showing the most. After a reducing agent was added to the samples, the vitamin C content in all samples increased (Figure 2). This clearly indicated the presence of the oxidized form of ascorbate that was not estimated when no reducing agent was present. While the vitamin C content in the squeezed juice could nearly be restored to its initial level that was not the case for the commercial juice. This indicates a decay of the molecule that cannot be restored as easily. Particularly, for the juice that was stored at room temperature next to a window, this seemed to be the case.
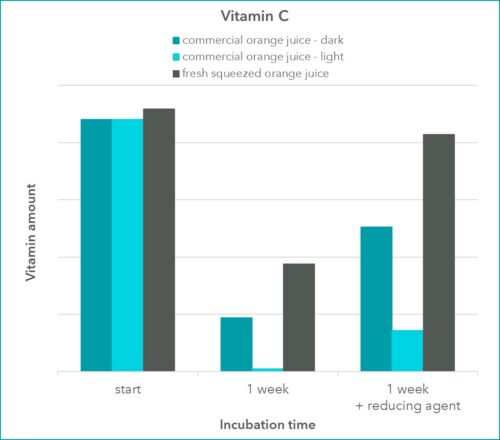
Figure 2: Amount of reduced Vitamin C in orange juice samples directly after opening or squeezing of a fruit, compared to the amount after storage and after storage plus treatment with a reducing agent during sample preparation. Each sample was measured as duplicate.
Conclusion
To determine the vitamin C content, it is important to know what will be measured. Here, we determined the amount of the reduced form of ascorbate. The differences after one week of storage show impressively that this form was not maintained throughout the experiment. Ascorbate can be oxidized quite fast however, the oxidized form can be regenerated to its reduced form with reducing agents. Still, the conversion did not lead to a complete reconstitution as compared to the initial values, indicating further molecular decay.
Again, to get the most out of your juice, drink it right away, no matter if its freshly squeezed or a commercial product.
Also, do not put a reducing agent inside your morning juice.






