Download this note (PDF)
Traditional mammalian cell culture is usually anchor-dependent and serum-supplemented. Animal-derived components tend to introduce lot-to-lot variability and blood-borne pathogens. Use of serum also tends to increase immunogenicity and production costs. Because of these issues, suspension cultures in animal-derived component free (ADCF) cell culture media are usually preferred for situations like recombinant protein production or viral vector/virus production due to its suitability to industrial processing and scale up. However, transitioning from adherent to suspension serum-free culture has its own set of challenges (see box) and is time consuming. Xell’s scientists have more than 20 years of experience with cell culture and state-of-the-art culture media. Adapting cell lines to serum-free suspension culture is just one of the many services we offer to the scientific community and biotechnology industry. Learn more about the do-or-die-approach/direct adaptation and the sequential approach and get a few helpful tips to get started with cell adaptation.
Figure 1: Infographic depicting general steps in the cell line adaptation process.
The “Do-or-Die” Approach or Direct Adaptation
- Expand the cultures like CHO K1 or HEK293 in currently used medium.
Tips to consider while trypsinization: If classical trypsin is used, inactivation or saturation of the enzyme with protein is necessary. If Accutase® is used, washing with serum-containing cell culture medium is not always required since it inactivates at 37 °C in about 45 minutes. - Centrifuge at 200 x g for 5 minutes a sufficient number of cells that will give at least 4-6 x 10⁵ cells/mL for inoculation. Discard supernatant.
Tips to consider during inoculation: It is not uncommon to observe a transient reduction in cell growth and viability during the course of the adaptation. Therefore, it is recommended to inoculate shake flasks with higher cell densities than usual. - Resuspend cells in Xell medium (if necessary, include 6-8 mM L-glutamine and/or growth factor).
- Passage cells or change medium every 2-4 days depending on cell density. Optimal cell density is dependent on the cell line and medium being used (see box).
Tips to consider during passage: Cells might still tend to aggregate. If you observe cell clumps while passaging, triturate them carefully (by pipetting up and down). Even if there is very little to no growth in the beginning, the medium should be exchanged completely each time. You can use a suitable pH-dependent dye (e.g. phenol-red) as a simple indicator for metabolic activity. When cells grow faster again, make sure to passage them well before they reach the stationary phase and adjust the splitting ratio when necessary. To make sure that no subpopulation with disadvantageous properties related to production will be selected, growth, productivity and product characteristics should be monitored carefully during the whole process. - Continue cell cultures until viability stabilizes at > 90% and growth rates remain constant over 3-5 passages.
- For optimal performance adapted cells should be inoculated at 2-5 ·10⁵ cells/mL in Xell cell culture medium. Cultures should be diluted every 2-4 days. Due to aggregation of cells, cultures should be stirred or shaken, using shake flasks, bioreactor systems or similar cultivation systems.
- Expand cultures like CHO K1 or HEK293 in currently used medium.
- Centrifugation similar to step 2 in the direct adaptation approach.
- Resuspend cells in Xell cell culture medium (if necessary, include 6-8 mM L-glutamine and/or growth factor) and 2-5% fetal bovine serum (FBS). Switch to a suspension system at this point.
- Refer to step 4 from direct approach for tips on passaging cells.
- Reduce serum concentration in the medium to 0.5-1% after at least three passages.
- Passage cells or change medium by centrifugation every 2-4 days depending on cell density.
- Reduce serum concentration in the cell culture media to 0% after 2-4 passages.
- Once cells have been shifted to medium with 0% serum, stable cultures are maintained as described in steps 5 and 6 of the direct approach.
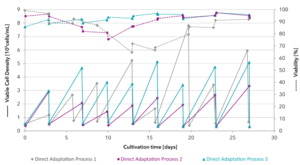
Figure 2: Direct adaptation process of HEK293 cells. Viable cell density and viability measurements during the direct adaptation process. Processes no. 1 to 3: (1) HEK 293 cell line A with Xell medium 1 (w/o FCS), (2) HEK 293 cell line B with Xell medium 2 (w/o FCS), (3) HEK 293 cell line B with Xell medium 3 (w/o FCS).
Tips to consider during cell adaptation: In order to save time and to increase your chances of success it might help to test different kinds of media in parallel during either cell adaptation process. See box for more challenges to consider while selecting media for cell adaptation.
Confused about the right media selection? We are here to help you make the right selection for your bioprocess.
When is the cell line adaptation complete?
As a rule of thumb, cells should grow stably in serum-free suspension culture for 3-5 passages with high viabilities.
How to choose between the Do-or-die Approach/ Direct Adaptation and Sequential Adaptation
When adapting cells to suspension culture and serum-free conditions you can either follow the do-or-die approach/direct adaptation we described above, or you can use the less harsh, but a more time-consuming, sequential adaptation procedure. Since suspension cultures can support higher cell densities (see box) you should want to use a richer medium that supports high cell densities and high product yields. You can either adapt them to the serum-free (e.g. surfactant-containing) version of the previously used basal cell culture medium like DMEM or RPMI and select a richer medium afterwards, or you can adapt them to the final medium directly (preferred approach). We recommend that it is best practice to start do-or-die approach/direct adaptation and sequential adaptation in parallel. After a couple of passages, it will become apparent whether the direct approach has been successful. If that fails having already started the sequential adaptation saves you time.
Challenges associated with transition from monolayer to serum-free suspension cultivation
Monolayers are largely limited by the available surface area, while in suspension culture, high cell densities can be reached. As a consequence, nutrient depletion and toxic metabolite accumulation often become the major limitations in suspension culture (also see Xell’s spent media analysis). Selection of a suitable, chemically defined culture medium, like our HEK ViP family of media, that is proven to support high cell densities, is an important first step. You still need to adapt your cells to three new situations:
- growth in suspension
- growth without serum
- growth in a new medium with different substrate or substrate concentrations
Cells grown in a shake flask or bioreactor are exposed to shear stress. The culture medium should therefore contain a suitable surface active and protective agent (surfactant). Xell’s cell culture media contain suitable surfactants that protect cells from shear stress. In addition, shaking frequency should be chosen carefully to reduce shear stress to a minimum.
Lack of serum exposes the cells to a range of stress like missing growth factors and other serum proteins. Albumin for example is an abundant serum protein that has the ability to bind and sequester a wide range of ligands, including ions and small molecules. Its absence can change the availability or dose-response relationship of certain cell culture media components. This has also an implication on the use of antibiotics. It is well-known that their activity is higher in serum-free media compared to those containing serum. If the use of antibiotics can’t be avoided, their concentration should be reduced (e.g. by 80 to 90%, needs to be tested individually).
Any form of additional stress during the adaptation, as for example induced by pH and temperature shifts, should be avoided.
Typical suspension cultivation conditions
Below you can find some typical cultivation parameters that we usually use for the suspension cultivation of various mammalian cell lines.
Typical parameters:
- shaking diameter: 5 cm
- shaking frequency: 125-185 rpm
- temperature: 37 °C
- CO2: 5%
Typical culture volumes:
- 125 mL, plain vent cap shaker: 20-50 mL
- 250 mL, plain vent cap shaker: 80-150 mL
- 500 mL, plain vent cap shaker: 200-300 mL
- 1000 mL, plain vent cap shaker: 400-600 mL
You don’t want to do it yourself? Then let us do it for you. Visit our webshop or contact us directly.






