Download this note (PDF)
Cysteine oxidation in cell culture media
Cell culture media typically provide all the amino acids necessary for cells to grow. Limitations in one or more amino acids do not only result in suboptimal growth of cell cultures, but also negatively impact product yield and quality. During process development, spent media analysis helps to monitor amino acid concentrations and determine consumption rates in order to detect and avoid such limitations.
This is particularly challenging in case of cysteine, as it gets easily oxidized to form cystine, a dimer of two cysteine molecules, connected via a disulfide bridge. Recently, a paper dedicated to temperature and storage stability of cell culture media also reported the amino acid cysteine to be particularly unstable under the conditions tested (Krattenmacher et al., 2018). This is in accordance with our experience, as we often measure no or only traces of cysteine, while cystine – its oxidized form – is clearly detected. We conducted experiments investigating the redox behavior of cysteine and cystine in water, bearing in mind that the more complex conditions found in (spent) cell culture media, might facilitate more complex chemistry.
Interconversion of cysteine to cystine in water
In order to learn more about cysteine (in-)stability we prepared a 1 mM solution in water, stored it at room temperature and took samples at different time points. We took several samples over the course of 26 h and then a “longterm” sample after 6 days. Each sample was derivatized directly after sampling and measured using our proprietary amino acid analysis gradient on an Agilent 1290 UHPLC-DAD system. A solution of 0.5 mM cystine was treated in the same way and served as a control.
As seen in Table 1 and Figure 1, even a sample of a freshly prepared cysteine solution (0 h) contained detectable amounts of cystine. Already 11% of the amino acid was initially present in form of the dimer. This value was quite stable for the first hours, possibly because the cysteine already contained some cystine before solubilization. After 24 h, more cysteine had shifted towards cystine resulting in 65% cysteine and 35% cystine. After 6 days, 98% of cysteine had been transformed to cystine (Figure 2).
In contrast to cysteine, cystine showed to be stable in solution. Its concentration stayed constant and no cysteine could be detected in any of the control samples (see Table 2, Figure 1+2).
In addition to the measurements described above, aliquots of the cysteine and cystine solutions were treated with DTT (dithiothreitol) or TCEP (tris(2-carboxyethyl)phosphine), two common reducing agents. As displayed in Tables 3 to 6, both reagents protected cysteine from oxidation to cystine, and even reduced cystine to free cysteine almost completely. Reducing effects of TCEP were stronger as compared to DTT.
Conclusion
Cysteine is a common constituent of cell culture media, but is often not detected in samples of spent media, because of its instability in aqueous solutions. The experiment described in this note shows that cysteine is mainly oxidized to form cystine. The oxidation of cysteine to cystine also implies that cysteine and cystine should not be included in the same standard mix. Instead, individual standards of cysteine could be prepared and protected from oxidation using TCEP.
Furthermore, we observed the oxidation of cysteine to proceed even faster in cell culture media spiked with additional cysteine (data not shown). This has to be considered, when potential amino acid limitations are being analyzed by spent media analysis. Hence, it is important to have appropriate quantitation methods in place.
Including cysteine and cystine, our method for amino acid analysis gives quantitative results for all proteinogenic amino acids as well as for ethanolamine, citrulline, hydroxyproline, ornithine and taurine. Contact us to request more information and test it.
Fast and reliable amino acid analysis
Tables and Figures
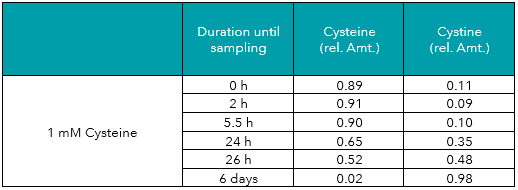
Tab. 1: Relative amounts (rel. Amt.) of cysteine and cystine in freshly prepared 1 mM cysteine in water, measured by UHPLC-DAD after derivatization. Sampling time is shown in hours/days after preparation.
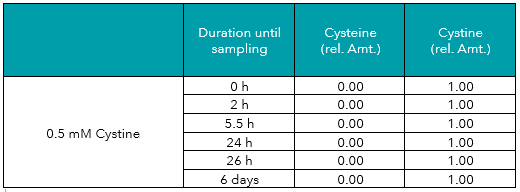
Tab. 2: Relative amounts (rel. Amt.) of cysteine and cystine in freshly prepared 0.5 mM cystine in water, measured by UHPLC-DAD after derivatization. Sampling time is shown in hours/days after preparation.
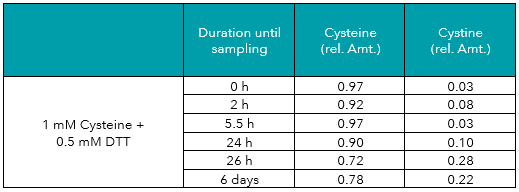
Tab. 3: Relative amounts (rel. Amt.) of cysteine and cystine in freshly prepared 1 mM cysteine plus 0.5 mM DTT in water, measured by UHPLC-DAD after derivatization. Sampling time is shown in hours/days after preparation.
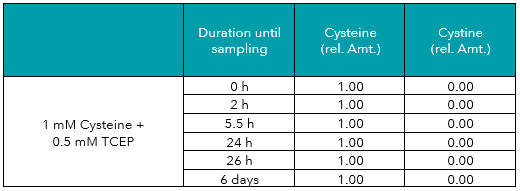
Tab.4: Relative amounts (rel. Amt.) of cysteine and cystine in freshly prepared 1 mM cysteine plus 0.5 mM TCEP in water, measured by UHPLC-DAD after derivatization. Sampling time is shown in hours/days after preparation.
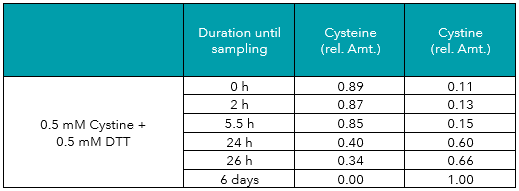
Tab.5: Relative amounts (rel. Amt.) of cysteine and cystine in freshly prepared 0.5 mM cystine plus 0.5 mM DTT in water, measured by UHPLC-DAD after derivatization. Sampling time is shown in hours/days after preparation.
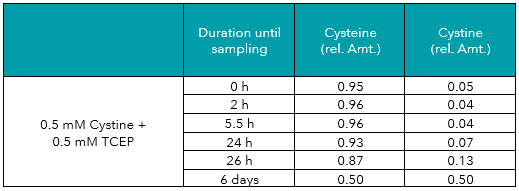
Tab.6: Relative amounts (rel. Amt.) of cysteine and cystine in freshly prepared 0.5 mM cystine plus 0.5 mM TCEP in water, measured by UHPLC-DAD after derivatization. Sampling time is shown in hours/days after preparation.
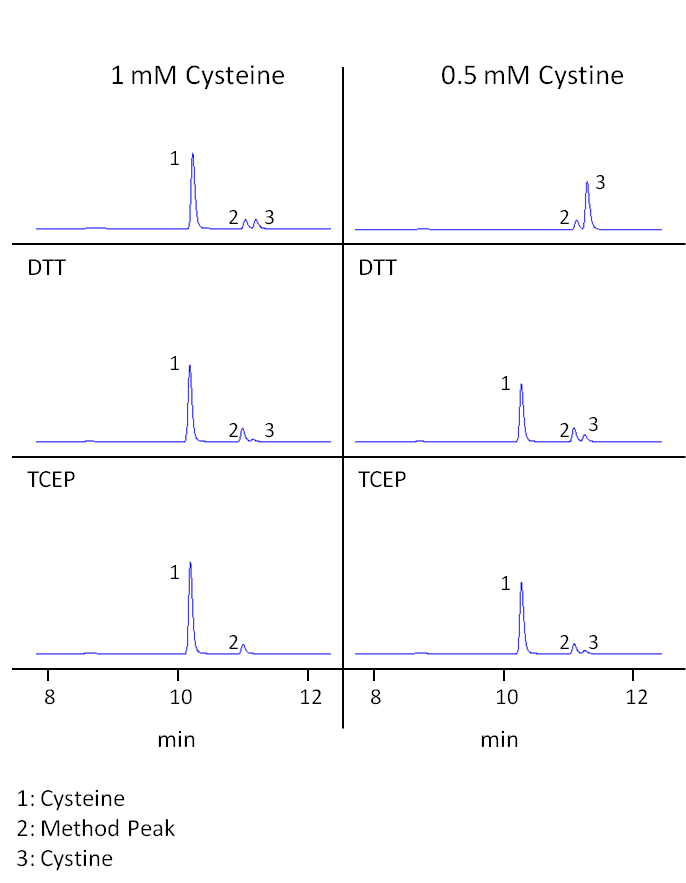
Fig. 1: Cysteine/cystine measurements 0h after preparation of the solutions. The first pane shows measurements without reducing agent, the second pane DTT reduction and the third pane TCEP reduction. Displayed are UHPLC-DAD chromatograms focusing on cysteine and cystine peaks measured after derivatization.
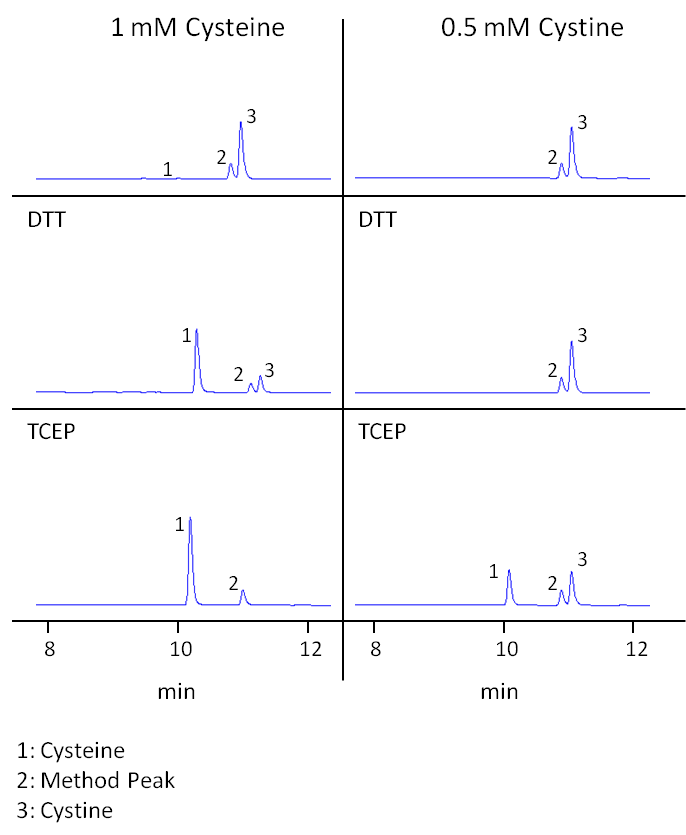
Fig. 2: Cysteine/cystine measurements 6d after preparation of the solutions. The first pane shows measurements without reducing agent, the second pane DTT reduction and the third pane TCEP reduction. Displayed are UHPLC-DAD chromatograms focusing on cysteine and cystine peaks measured after derivatization.






