Adeno-Associated Viral vectors (AAV) are among the most widely used vectors for gene therapy applications and their importance has become even more evident recently. To support you in your process scale-up, we address questions like “Why should you consider transferring your AAV production from adherent to suspension?” and discuss different case studies (AAV-2 and AAV-8) in our technical note. You can read and download it here.
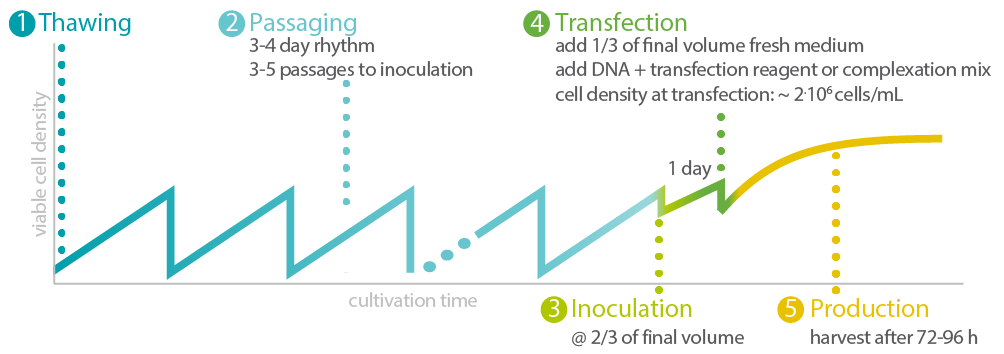
Exemplary procedure for seed-train and production process. See the actual transfection and AAV-2/ AAV-8 production data here.
To boost your AAV manufacturing process instantly, request your sample of our HEK ViP NX medium – chemically defined and optimized for AAV production






