Portrait:
Research and Development Department
More than 20 years of experience in cell culture technology – get to know your ideal partner
Media Development | Process Development | Suspension Adaptation | Cell Studies | many more
As a leading company for custom cell culture media and feed development, Xell is your ideal partner for solutions that meet the individual needs of your cell line and enable them to deliver the highest product yields. Depending on the application and in close collaboration with you, we carefully choose individual target values for process performance. Following a streamlined workflow, we combine powerful spent media analysis with intelligent DoE and process simulations in order to reach your objectives in the most efficient way.
Since our company was founded in 2009, our focus has always been on the development of innovative cell culture media. So together with our experienced employees, we work hard to keep building on our knowledge and experience and to expand our research and development department. We are open to every cell culture-related project you may have – from small-scale R&D to large-scale projects for global players. Our key ingredients for a successful collaboration are:
- Long-standing experience in cell culture technology and medium development
- Dedicated team of highly trained professionals with academic backgrounds
- Extreme flexibility in setting up your custom project
- Short timelines and direct support whenever you need it
- Complementary services: from cells, processes and analytics to media development and manufacturing
State-of-the-art equipment and methods
Our BSL1 and BSL2 laboratories are well equipped to support any cell culture project requirements and are constantly upgraded with regard to new technologies, methods and devices to provide you with state-of-the-art services and results.
- Incubators and shakers for adherent and suspension cell culture
- Bioreactor systems in different scales (0.8 L, 2 L, 5 L, 10 L)
- Automated and semi-automated cell-counters, plate imager
- Instruments and methods for at-line analytics (e.g., pH, osmolality, redox, glucose, lactate, ammonia, LDH)
- PCR, qPCR, ddPCR for product or cell analysis
- Flow cytometry
- Diverse spent media analytics through our in-house analytics department (e.g., amino acids, vitamins, trace elements)
Certification and compliance
Xell’s quality management system is fully compliant with DIN EN ISO 9001 and is reexamined on a yearly basis and certified by an external, independent agency. Our research, project management and workflows are fully compliant with DIN EN ISO 9001. If you want to receive further information, to learn more about Xell’s quality management system and the application of our products and services for GMP manufacturing, or to evaluate a remote or on-site audit, please contact us or visit our quality and compliance section.
Our collaborative team of experts leverages powerful tools, models and platforms to ensure that Xell effectively and efficiently meets all its various targets. At the same time, it makes sure that any project-related customer needs are successfully met. Close communication and a streamlined workflow enable us to realize any project in a fast and very flexible manner.
No matter the scale or aim of your project, ranging from brief consulting to complex medium or process development – through our close cooperation and flexibility, we are able to support you in any way, including through the provision of services that are not listed on this homepage – just reach out to discuss your plans with us.
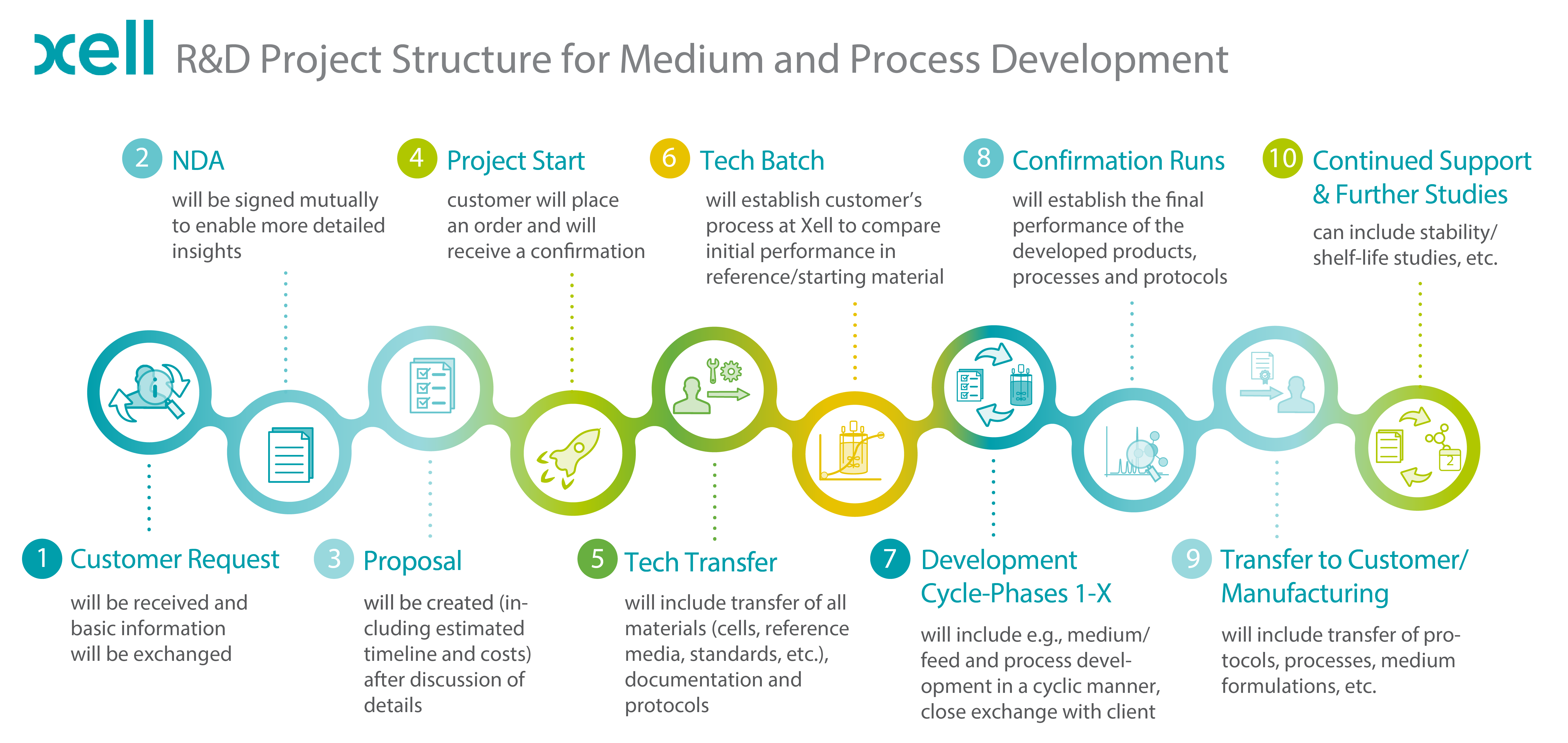
FIGURE: General project structure of CRO projects at Xell.
Service Portfolio
Our intensive work in the field of mammalian cell culture processes makes us an excellent partner for solving your cell culture process challenges.
With our flexible and customer-focused approach, we provide efficient concepts and supply capacities to realize your research projects. To optimize and streamline your process, we apply our knowledge platform and make use of our broad on-site analytics department. Please contact us for any project challenges you wish to discuss.
Make use of our complete complementary portfolio to maximize the optimization potential of your project. Depending on your timeline and budget, we will suggest different levels of involvement to find the best fitting solution. We are happy to advise you individually.
Traditional mammalian cell culture is usually anchor-dependent and serum-supplemented, which directly results in a series of disadvantages due to the variability caused by serum/animal-derived components. In contrast, suspension cultures in animal-derived component free (ADCF) cell culture media have several advantages:
- reduced lot-to-lot variability of the medium
- easier scale-up
- no risk of blood-borne pathogens
- massively reduced production costs
- increased animal welfare
Because of these advantages, suspension cultures in animal-derived component free (ADCF) cell culture media are usually preferred for situations like recombinant protein production or viral vector and virus production due to their suitability for industrial processing and scale-up. However, transitioning from an adherent to suspension serum-free culture has its own set of challenges and is time consuming.
Xell’s scientists have more than 20 years of experience with cell culture and state-of-the-art culture media. Adapting cell lines to serum-free suspension cultures is just one of the many services we offer to the scientific community and biotechnology industry. We adapt your adherently growing cell line (suspension) for growth in a suitable serum-free, chemically defined cell culture medium of your choice. For more insight, feel free to read our technical note on this topic.
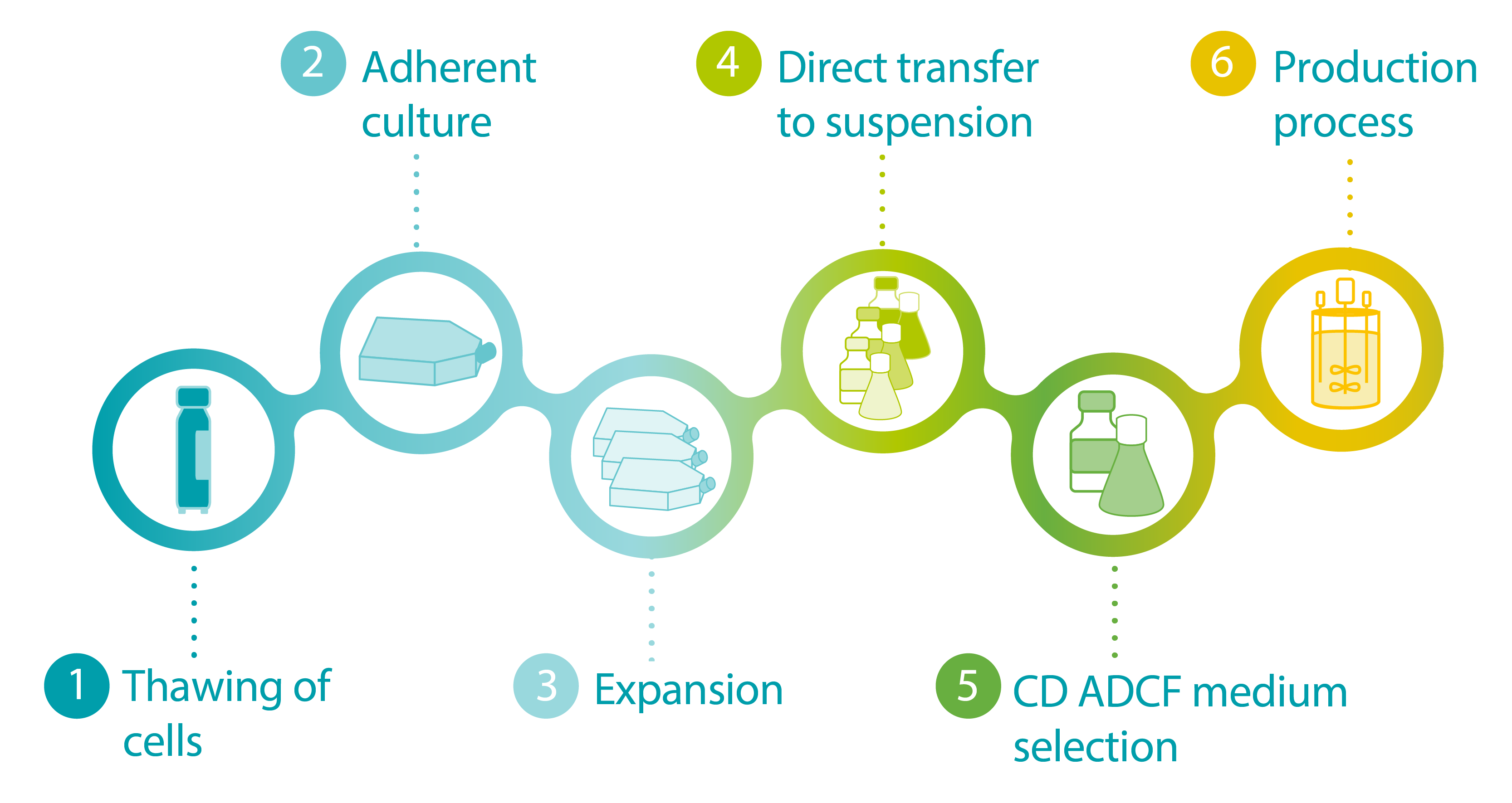
FIGURE: Infographic depicting general steps in the cell line adaptation process.
With our broad range of analytical methods (cell growth, product quantification and qualification, flow cytometry applications, qPCR/ddPCR), we offer detailed characterization of your cell lines. Cell line stability and selection pressure are potential issues to be addressed. Depending on the culture medium, the concentration of selection reagents may need to be adapted to guarantee stable production while maintaining high process performance. Furthermore, we recommend the evaluation of long-term stability which can be critical when extended seed-train periods are necessary.
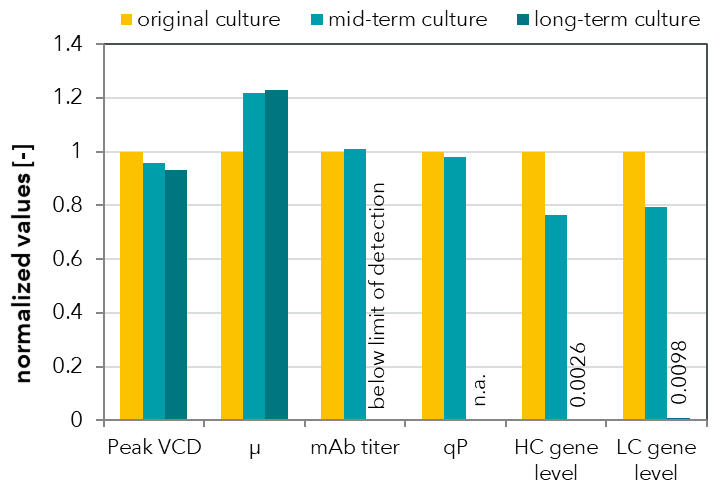
FIGURE 1: Comparison of process values for a monoclonal antibody (mAb)-producing CHO cell line at three time points over a high passage experiment. Peak viable cell density was measured in bioreactor batch cultivations. mAb titer was determined by HPLC and relative gene levels were measured by quantitative real-time PCR. Loss of productivity was detected in long-term culture and was attributed to product gene loss.

FIGURE 2: Flow cytometric analysis of a CHO cell line cultivated in a high passage experiment. Heavy and light antibody chains were measured after intracellular staining with fluorescently labeled antibodies. A subpopulation with no HC expression was already detected in the mid-term culture. The long-term culture exhibited no HC expression and reduced LC expression. These results are reflected by the HPLC measurements of mAb titers (see FIGURE 1).
With our key competences in medium development and manufacture, we know that raw materials (production, origin, quality) and production technology are crucial factors in influencing the quality of the final medium product. As a result, the same medium formulation can lead to deviating process performances (see FIGURES 2 and 3). We therefore offer to analyze and test raw material sources and to compare different suppliers. Furthermore, we recommend the respective biological testing of potential raw materials and the evaluation of products from different suppliers with regard to process performance and production. Powder and liquid media characterization as well as solubility studies of your products are also part of our portfolio.
For a more detailed example of our raw material analysis skills, read our technical note on trace element impurities in amino acids of different suppliers .
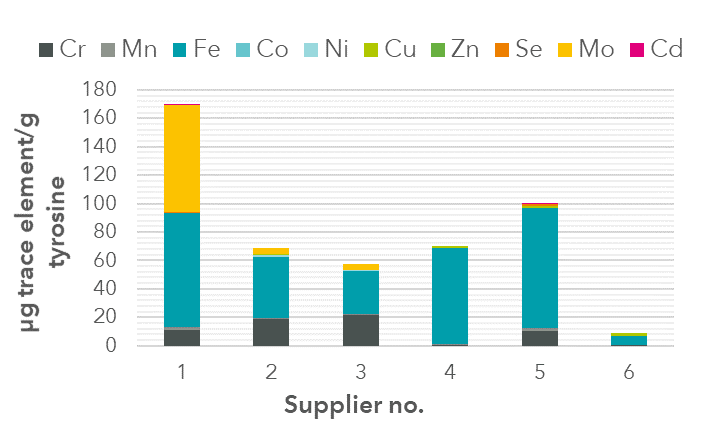
FIGURE 1: Impurities found in tyrosine samples from different suppliers. The greatest impurities occurred with: Iron (Fe), Molybdenum (Mo) and Chromium (Cr).
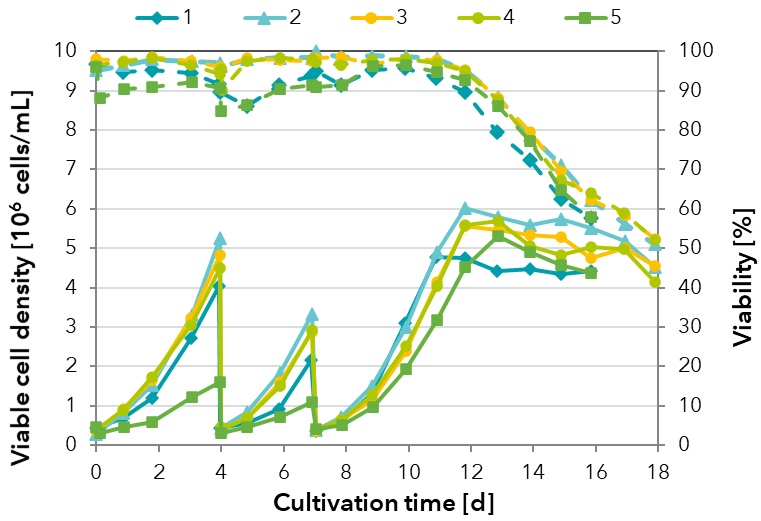
FIGURE 2: Comparison of the growth performance of a HEK cell line in pre-culture passaging and batch cultivation with one medium formulation containing different variants (1-5) of one raw material for each culture.
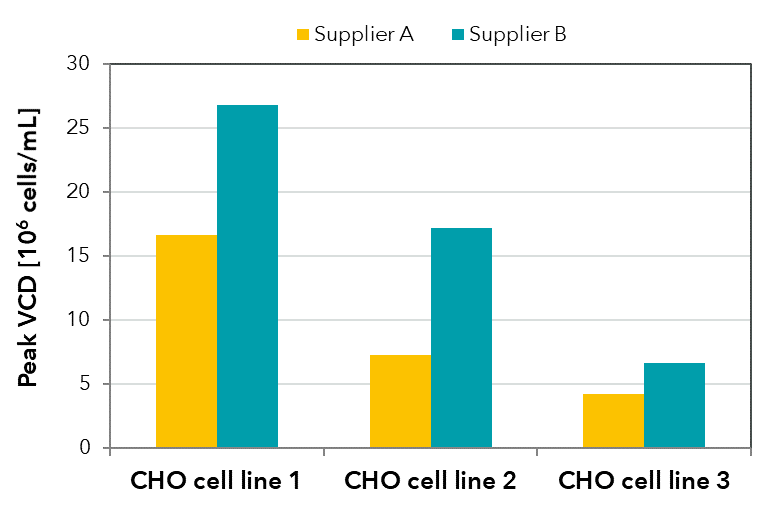
FIGURE 3: Peak viable cell densities achieved in batch shaker cultivations with the same medium formulation produced by two different suppliers for three CHO cell lines.
Lot-to-lot variation is a well-known effect in media production and can occur even in chemically defined media. It can be caused, for example, through a change of raw materials or as a result of minor changes in the powder production process or the solubilization procedure. Furthermore, lot-to-lot variation can occur due to impurities in raw materials. Components such as amino acids in USP/pharma grade with ≥ 98-99% purity may still contain foreign amino acids and trace metals (see our technical note on trace element impurities). To evaluate variation in your media lots, we highly recommend biological performance tests and respective analytics. For this, we can establish and perform routine lot testing (see FIGURE).
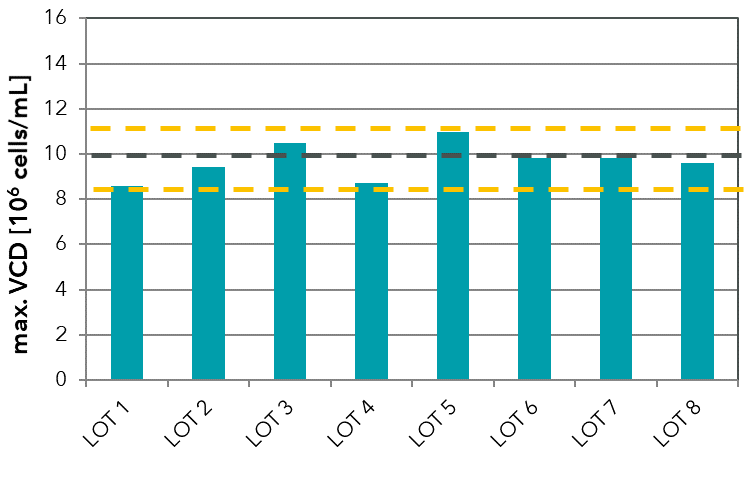
FIGURE: In-house testing of different medium lots of a chemically defined medium formulation developed by Xell. The test was performed with a CHO cell line to define thresholds for biological performance testing of the powder lots.
Process development, such as feed strategy optimization, can take up a lot of time and resources. With our long-standing experience in cell culture technologies and medium development, we can provide process development services including:
- Development/optimization of a feeding strategy
- Metabolic optimization of feeding
- Optimization of seed trains and handling procedures
- Parameter profiles, e.g., pH, temperature and DO
- Development/optimization of perfusion processes
View our exemplary project.
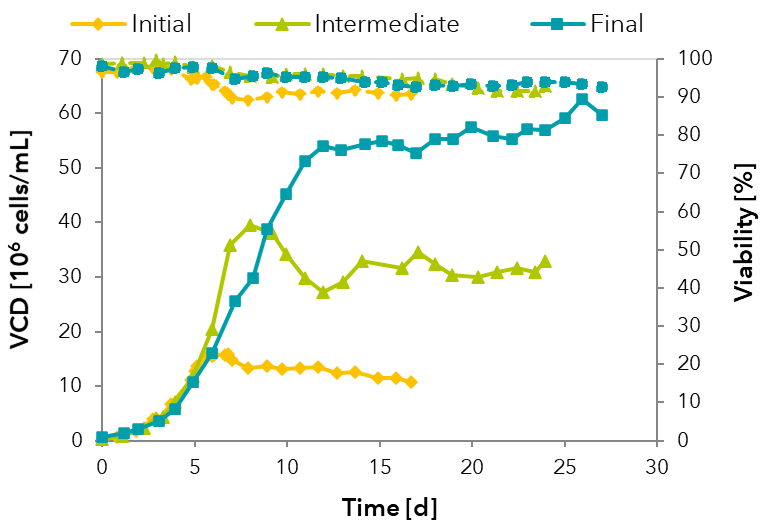
FIGURE: Optimization of a perfusion process from initial to final process.
Cell culture medium is a liquid designed to support the growth of cells in an artificial environment. Usually, it contains a mixture of:
- an energy source like carbohydrates in the form of sugars
- amino acids since they are the building blocks of proteins
- fatty acids and lipids – particularly important in serum-free media
- inorganic salts to maintain osmotic balance
- vitamins and trace elements essential for the growth and proliferation of cells
- buffering system to maintain the pH in the physiological range
Cell culture feed is a liquid supplement that is mainly used in fed-batch cell culture processes and supports superior production by increasing the final cell density and extending the production capability of the cultures compared to batch process. It contains highly concentrated nutrients to increase the productivity of cultured cells. Consumed substances like vitamins and amino acids are replenished to extend the process and thereby increase product yield.
Selecting the best matching medium and feed for your cells is highly essential as it is the easiest way to generate maximum product yields. However, choosing the best medium can be quite a challenge and requires a lot of knowledge, not only about the cell’s requirements but also about the available options.
The selection process typically involves screening and benchmarking alternative off-the-shelf culture media from several suppliers. With our long-standing experience in cell culture technologies and medium development, we can provide expertise and resources for these routine studies. We will include any chemically defined media of your choice, not limited to media produced by Xell.
If you are just beginning to set up a cell culture lab or you are experiencing process limitations, problems in your scale-up or perhaps seeing batch-to-batch variations in your current medium, we are your trusted partner for scientific consulting. With years of experience in cell culture technologies, medium development and medium production in liquid and powder form, we deliver rapid and targeted support for your challenges. For example, we can work with your in-house spent media data to help optimize a process or feeding strategy. Consulting can also include on-site support if required.
Medium and Feed Development
Xell offers targeted, rapid and efficient services for the development of custom cell culture media and custom feed supplements.
We have previously developed custom cell culture media and feeds for cell lines like CHO, BHK, hybridoma, HEK and other human cell lines throughout the biotech industry (view our commercial references. Individual and highly efficient, these customized solutions represent the essence of Xell’s expertise. All of our media and feed products are chemically defined, animal component-free and available as liquid or powder formulations.
Custom medium development can be planned and managed according to your needs and timeline. With our long-standing experience, we can support your processes for applications such as:
- Production of diagnostic and therapeutic proteins
- Transient gene expression
- Vaccine production
- Gene/cell therapy applications from AAV to Lentivirus (Technical note: The journey to AAV production)
- Novel food applications/sustainable meat (biomass, cells, media)
To discuss your process challenges and take advantage of a free feasibility study to evaluate the potential of custom development now – contact us.
Gene Therapy and Related Products/Services
Whatever your gene therapy project targets, let us help you exceed them!
Gene therapy has become the focus of worldwide research and has gained the intense attention of many active research groups, universities and biotech and pharmaceutical companies. But what exactly is gene therapy? The term gene therapy refers to the insertion or replacement of nucleic acids (e.g., DNA or RNA) into animal cells (gene editing) in order to treat genetic diseases or tumors, for example. The most commonly used vectors in DNA/RNA delivery are adeno-associated viruses (AAVs) or lentiviruses which can be produced in cell cultures after transfection, induction or infection.
Here at Xell, we are dealing extensively with the question of how to optimally produce viruses and viral vectors and we have already developed suitable cell culture media and processes in this context to maximize production yields. Moreover, we are constantly working on optimized methods for processes and analytics to advance this topic and are happy to support our customers in making the most of their projects. This includes, for example, the use of the newest and most efficient transfection reagents on the market and tailoring our media and processes to them.
Our services related to gene therapy-based production include:
- Proprietary off-the-shelf media and feed solutions for HEK and other human cell lines
- Development of customized media and feed for viral vector production
- Liquid and powder medium manufacture
- Suspension adaptation service and transfer of the complete process from adherent to suspension
- Process development and optimization, including transfection protocols
- Product analytics from qPCR/ddPCR/ELISA
- Method development for further analytics and purification
- In-depth analysis of spent media to better understand your cells and processes

FIGURE: Gene Therapy and related Products/Services at Xell
Exemplary Projects
Gain first insights into our scientific mindset and view our exemplary projects.
Exemplary projects depict Xell’s scientific approach, starting with making hypotheses, creating predictions and drawing logical consequences before applying them to an optimized experimental approach. Drawing from our analytical capacities and utilizing our vast experience in cell culture technology, we analyze the results and draw sound conclusions that are transformed into unique solutions for our customers’ challenges.
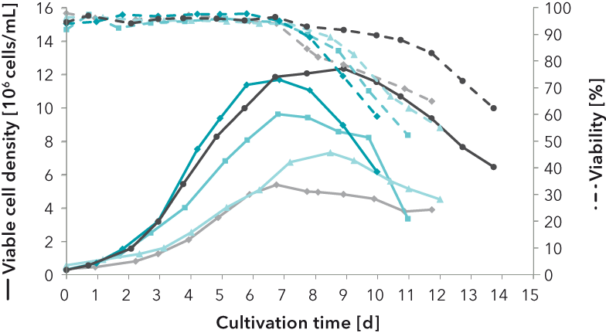
FIGURE 1: Development of a platform medium for a protein-producing CHO cell line. Viable cell density (VCD) and viability of batch and fed-batch cultivations in shaking flask and in bioreactor scale are compared using a commercially available reference medium, a developed interstage medium and the developed platform medium. The latter provides an excellent basis for further fast and efficient development steps.
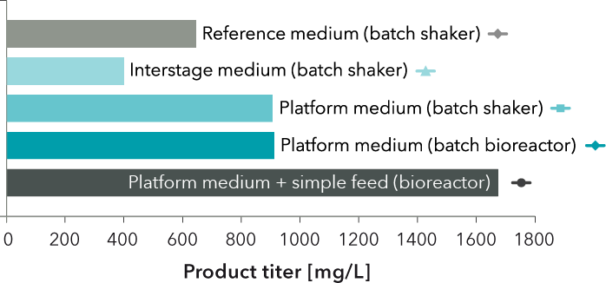
FIGURE 2: Maximum product titer for the cultures shown in FIGURE 1. Titer in platform medium was 1.4 times greater for batch cultivations and 2.6 times greater for a bioreactor process with a simple feed compared to reference cultivation.
Xell’s customized medium and feed solutions were applied in a GMP-production of material for pre-clinical and phase I clinical trials. The therapeutic recombinant fusion protein was produced in fed-batch processes with a CHO cell clone. Process development was performed in 2 L scale whereas the production process for clinical material was accomplished in a 100 L stirred tank bioreactor.
Cultivations were performed under standard conditions. A temperature shift was initialized when cells entered the stationary phase. Upscaling was successful and reproducible growth performance could be demonstrated for a cultivation of 15 days (see FIGURE 1). The peak viable cell density at day 6 decreased slightly within the stationary phase due to volume increase by feeding. Consequently, very high viabilities were maintained throughout cultivation, dropping marginally below 90% at day 15. Product formation of the fusion protein was monitored by RPHPLC. In both scales, comparable yields were detected (see FIGURE 2).
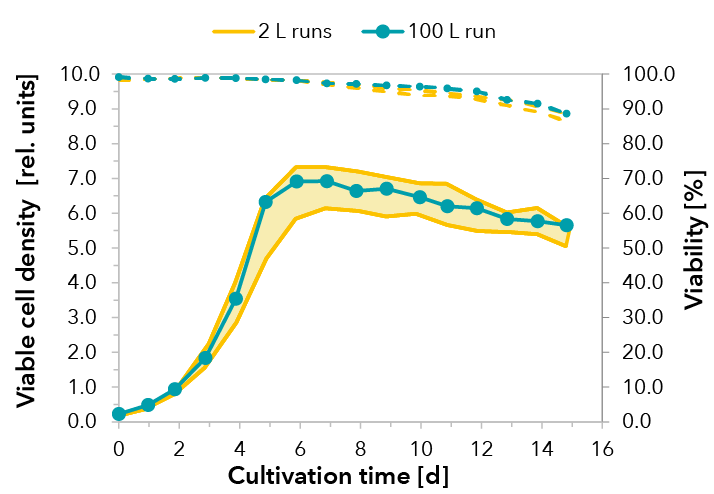
FIGURE 1: Viable cell density and cell viability in the 100 L production process run are comparable to runs from process development in 2 L scale, demonstrating the successful upscaling of the process. The shaded area illustrates the results of three independent 2 L runs.
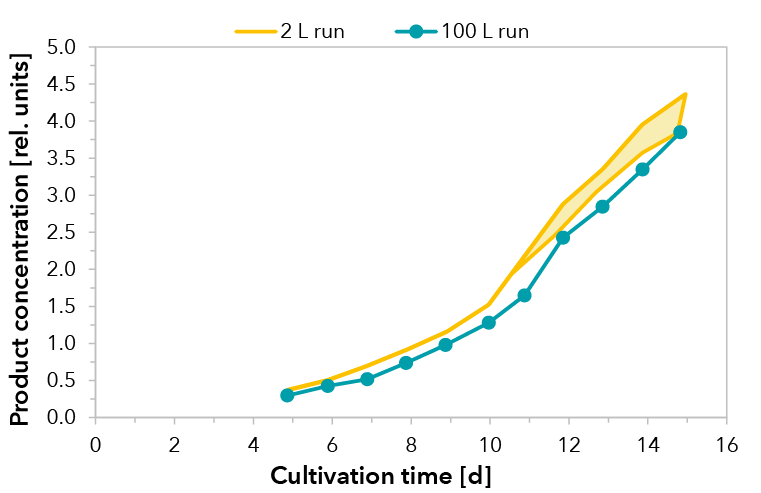
FIGURE 2: Relative product concentration for the 100 L scale production process run and runs from process development in 2 L scale (shaded area, n=3).